Clinical Risk Assessment Approvals
Why automate?
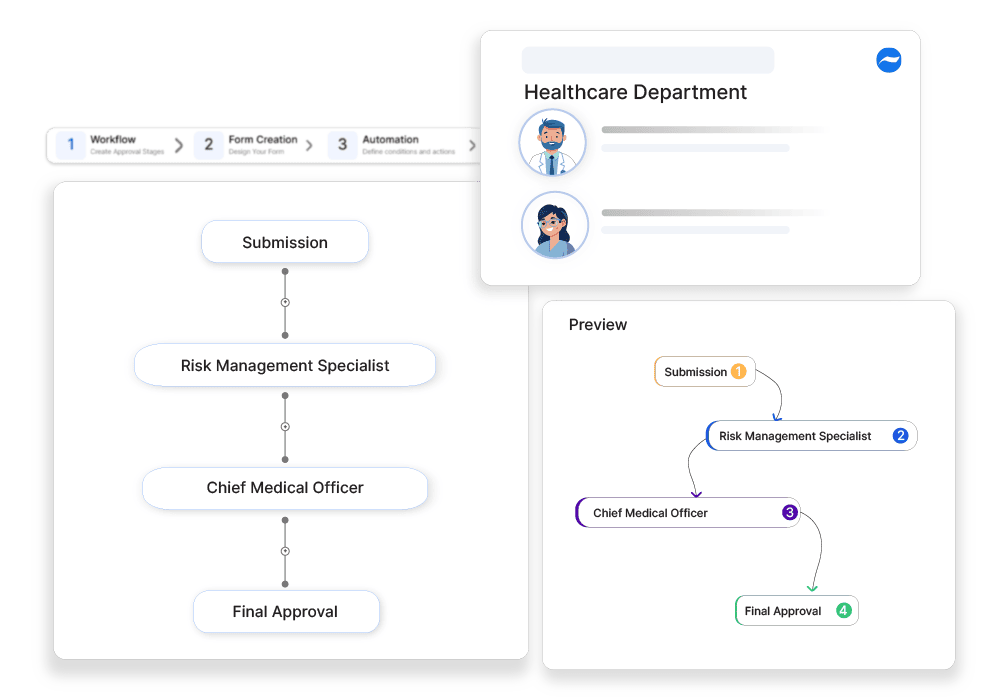
How Cflow Can Help Automate the Process:
Automated Assessment Workflow:
Cflow automates the entire workflow for clinical risk assessments, ensuring that each assessment is routed through the necessary stages for evaluation and approval. This reduces manual intervention and accelerates the approval process.
Customizable Risk Criteria:
With Cflow, healthcare organizations can define custom risk criteria tailored to their specific needs. This ensures that each assessment meets the required standards before approval, maintaining high care quality.
Real-time Tracking and Notifications:
Cflow offers real-time tracking of risk assessment approvals, allowing stakeholders to monitor the status of each assessment. Automated notifications are sent to relevant personnel, ensuring timely action and reducing the risk of delays.
Comprehensive Documentation:
Cflow maintains detailed records of all risk assessment approvals, including submission details, evaluation actions, and reviewer comments. This comprehensive documentation supports regulatory compliance and provides valuable insights for future reference.
Frequently Asked Questions
What factors are considered in clinical risk assessments?
Patient safety, potential adverse effects, and compliance with medical protocols.
Who is responsible for conducting a clinical risk assessment?
Risk management teams, medical committees, and regulatory compliance officers.
What happens if a clinical risk assessment identifies high risk?
Risk mitigation strategies are implemented, and treatments or procedures may be revised.



