Clinical Trial Data Access Requests
Why automate?
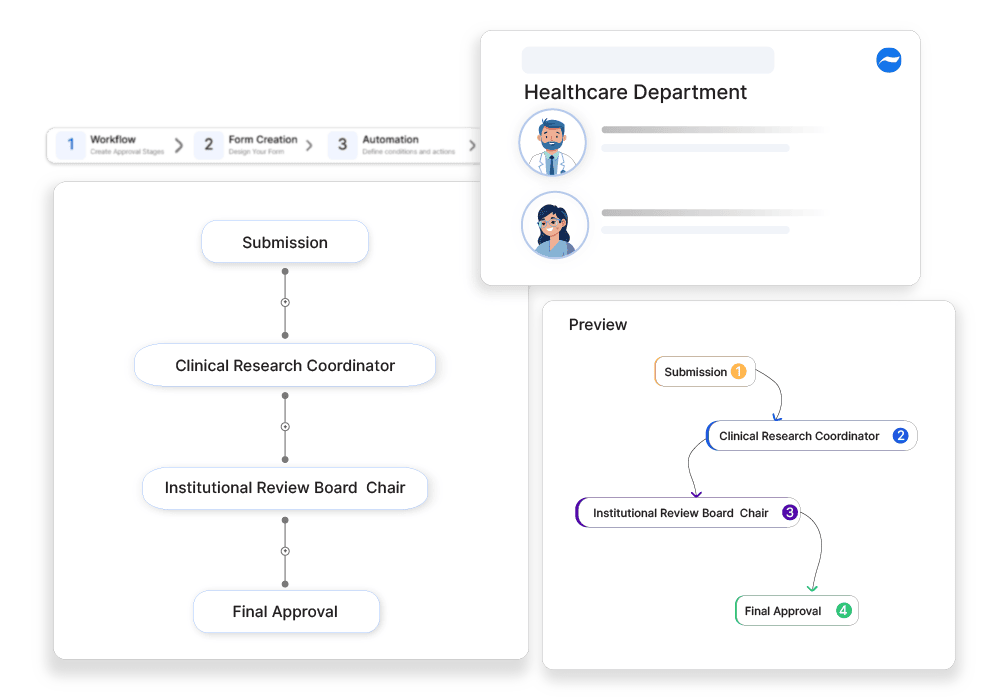
How Cflow Can Help Automate the Process:
Efficient Request Submission:
Cflows customizable access request forms capture all necessary information about clinical trial data and researcher credentials accurately, reducing the time spent on manual data entry and ensuring completeness from the start.
Real-Time Communication:
With Cflow, researchers and relevant departments receive real-time notifications when an access request is approved or requires additional information, ensuring timely communication and reducing delays.
Enhanced Data Security:
By automating the clinical trial data access request process, Cflow enhances data security by ensuring that data is accessed securely and in compliance with regulatory requirements, improving research timelines and outcomes.
Automated Verification:
Cflow can automatically verify researcher credentials and data access requirements, minimizing the burden on administrative staff and speeding up the approval process.
Frequently Asked Questions
Who is eligible to access clinical trial data?
Researchers, regulatory bodies, and healthcare institutions with proper authorization.
What security measures protect clinical trial data?
Data encryption, controlled user access, and anonymization of patient records.
How long is clinical trial data retained?
Typically for 5-15 years, depending on regulatory guidelines.



